


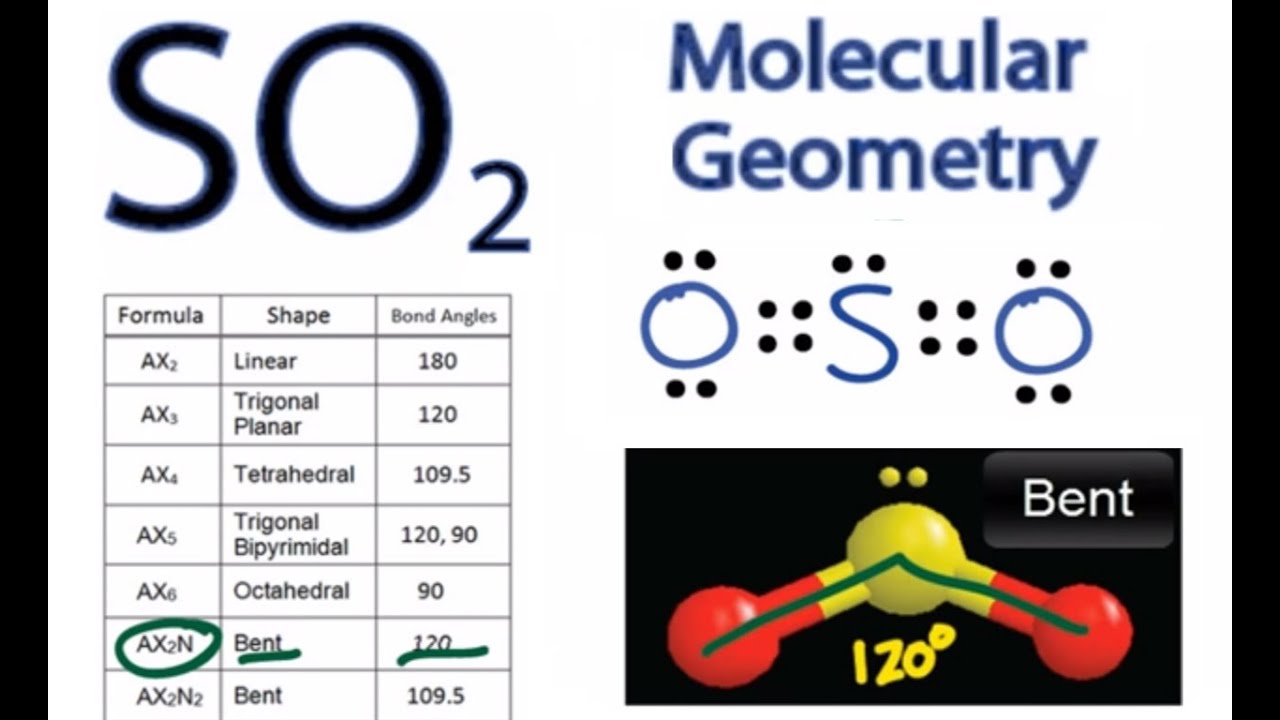
We currently do not have a feature to support diagrams/graphs and will be updating soon. The above discussion explains the electron-pair geometry and molecular geometry of a water molecule. There is also lone pair-lone pair repulsion that occurs between the 2 lone pairs of electrons present on the central O atom so due to this reason, the two bond pairs acquire a bent shape. On the other hand, only the 2 bond pairs (2 O-H bonds) are considered in the molecular geometry of H₂O. In the electron-pair geometry of H₂O, there are a total of 4 electron groups (2 bond pairs and 2 lone pairs of electrons). Also, 2 lone pairs of electrons are present on the central O atom. In the structure of a water (H₂O) molecule, it is observed that the central atom is an oxygen (O) atom and it is attached to 2 Hydrogen (H) atoms. No lone pairs of electrons are considered in the case of molecular geometry. The electron-pair geometry deals with the arrangement of all the electron groups present (considers both bond and lone pairs of electrons) whereas molecular geometry is determined when only bond electron pairs are considered. There is a difference between electron-pair geometry and the molecular shape of a molecule. Biological Science (Freeman Scott Quillin Kim Allison Lizabeth).Brunner and Suddarth's Textbook of Medical-Surgical Nursing (Janice L.Interpersonal Communication (Kory Floyd).Give Me Liberty!: an American History (Eric Foner).
#Electron pair geometry professional
Professional Capstone Project (PSY-495).Professional Application in Service Learning I (LDR-461).Professional Nursing Practicum (NUR - 4836C).Concepts of Medical Surgical Nursing (NUR 170).Leading in Today's Dynamic Contexts (BUS 5411).Anatomy & Physiology I With Lab (BIOS-251).Managing Engaging Learning Environments (D095).Organic Chemistry Laboratory I (CHEM 233).Operating Systems 1 (proctored course) (CS 2301).Management in Global Economy (BUS 5211).


 0 kommentar(er)
0 kommentar(er)
